
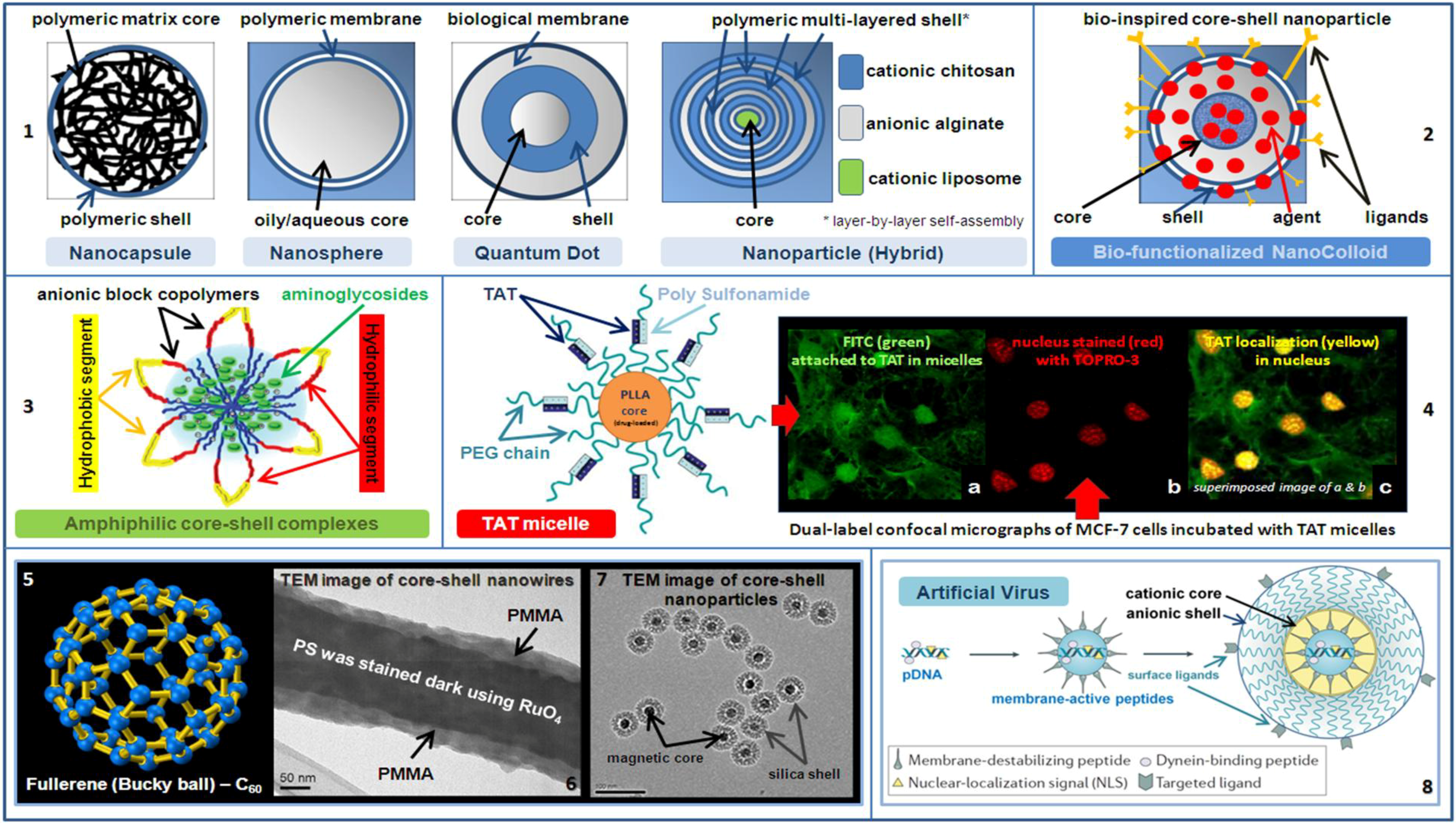
18 Such active targeting strategies can not only effectively deliver drugs into cancer cells, but also reduce the amounts of anticancer agents used. 15– 17 For example, asialoglycoprotein receptors are located on liver cancer cells and can specifically bind with glycoprotein on/in galactose residues, leading to internalization and subsequent degradation of glycoprotein inside the cells. Specific ligands (eg, folate) that can be recognized by cancer cells have been widely studied and linked to the surfaces of anticancer agents so that receptor-mediated endocytosis can occur. 13, 14 In contrast with passive targeting, active targeting has attracted more attention in terms of further enhancing delivery efficacy and cancer specificity. 12 However, such passive targeting-based drug delivery systems sometimes cause unwanted accumulation of anticancer agents in the liver, kidney, and spleen. The fabrication of nanoscale drug-loading carriers has been widely studied because these nanoparticles can accumulate in tumor sites via the enhanced permeability and retention effect. Most anticancer drugs cannot distinguish between cancer cells and healthy cells, which results in undesirable side effects and low efficacy.
#Functionalized core shell nanoparticles how to
One of the challenges for effective cancer therapy is how to deliver therapeutic agents to tumor sites. 11 Consequently, hyperthermia is an effective and safe cancer therapy. Thus, this increases the possibility of recognition of cancer cells by cytotoxic T lymphocytes. 10 In addition, studies have also shown that cancer cells produce heat shock proteins, resulting in an increase in major histocompatibility complex class I antigens. 8, 9 When a high temperature (usually around 42.5☌) is produced locally, tumor tissue cannot release heat energy via the bloodstream due to dysfunction of blood vessels. The reason is that cancer cells are more sensitive to heat than normal cells. 7 The hyperthermia of SPIONs has been considered as one of the most promising cancer therapies because it can effectively destroy cancer cells while keeping normal cells intact. Among these applications, hyperthermia 6 is a physical therapy in which SPIONs generate heat and thus kill cancer cells when they are exposed to an external magnetic field. Superparamagnetic iron oxide nanoparticles (SPIONs), including magnetite (Fe 3O 4) and maghemite (γ-Fe 2O 3), have been extensively utilized in biomedical applications, including as magnetic resonance imaging contrast agents, 1 for biocatalysis, 2 for biological separation, 3 for biosensing, 4 diagnostic medical devices, 5 and hyperthermia. Keywords: hyperthermia, iron oxide, alginate, pre-gel, targeting After treatment with an AC magnetic field, the results indicate that Fe 3O nanoparticles had excellent hyperthermic efficacy in a human hepatocellular carcinoma cell line (HepG2) owing to enhanced cellular uptake, and show great potential as therapeutic agents for future in vivo drug delivery systems. Here a new core-shell nanostructure consisting of inorganic iron oxide (Fe 3O 4) nanoparticles as the core, organic alginate as the shell, and cell-targeting ligands (ie, D-galactosamine) decorated on the outer surface (denoted as Fe 3O nanoparticles) was prepared using a combination of a pre-gel method and coprecipitation in aqueous solution. However, the development of a magnetic fluid agent that can selectively target a tumor and efficiently elevate temperature while exhibiting excellent biocompatibility still remains challenging. Shih-Hsiang Liao, 1 Chia-Hung Liu, 2 Bishnu Prasad Bastakoti, 3 Norihiro Suzuki, 7 Yung Chang, 4 Yusuke Yamauchi, 3 Feng-Huei Lin, 5,6 Kevin C-W Wu 1,6ġDepartment of Chemical Engineering, National Taiwan University No 1, Taipei, 2Department of Urology, Taipei Medical University-Shuang Ho Hospital, New Taipei City, Taiwan 3National Institute for Materials Science, Ibaraki, Japan 4R&D Center for Membrane Technology and Department of Chemical Engineering, Chung Yuan Christian University, Taoyua, 5Institute of Biomedical Engineering, National Taiwan University No 1, Taipei City, 6Division of Medical Engineering Research, National Health Research Institutes, Miaoli County, Taiwan, 7International Center for Young Scientists (ICYS), National Institute for Materials Science (NIMS), Tsukuba, Ibaraki, JapanĪbstract: Hyperthermia is one of the promising treatments for cancer therapy.


 0 kommentar(er)
0 kommentar(er)
